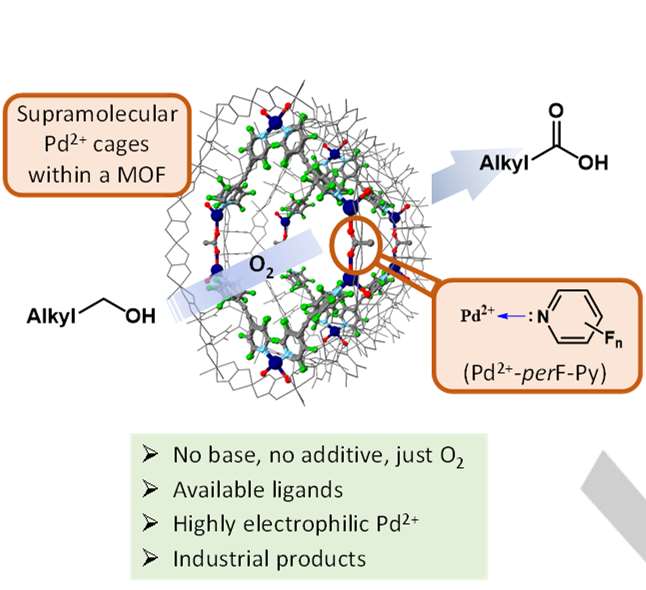
Extremely high electrophilic metal complexes, composed
by a metal cation and very electron poor –donor ancillary ligands,
are expected to be privileged catalysts for oxidation reactions in
organic chemistry. However, their low lifetime prevents any use in
catalysis. Here we show the synthesis of fluorinated pyridine–Pd2+
coordinate cages within the channels of an anionic tridimensional
metal organic framework (MOF), and their use as efficient metal
catalysts for the aerobic oxidation of aliphatic alcohols to carboxylic
acids without any additive. Mechanistic studies strongly support that
the MOF–stabilized coordination cage with perfluorinated ligands
unleashes the full electrophilic potential of Pd2+ to dehydrogenate
primary alcohols, without any base, and also to activate O2 for the
radical oxidation to the aldehyde intermediate. This study opens the
door to design catalytic perfluorinated complexes for challenging
organic transformations, where an extremely high electrophilic metal
site is required.