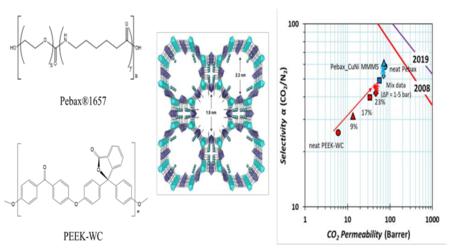
Mixed matrix membranes (MMMs) are seen as promising candidates to overcome the fundamental limit of polymeric membranes, known as the so-called Robeson upper bound, which defines the best compromise between permeability and selectivity of neat polymeric membranes. To overcome this limit, the permeability of the filler particles in the MMM must be carefully matched with that of the polymer matrix. The present work shows that it is not sufficient to match only the permeability of the polymer and the dispersed phase, but that one should consider also the individual contributions of the diffusivity and the solubility of the gas in both components. Here we compare the gas transport performance of two different MMMs, containing the metal organic framework CuNi-MOF in the rubbery Pebax®1657 and in the glassy poly(ether-ether-ketone) with cardo moiety, PEEK-WC. The chemical and structural properties of MMMs were investigated by means of FT-IR spectroscopy, scanning electron microscopy and EDX analysis. The influence of MOF on the mechanical and thermal properties of both polymers was investigated by tensile tests and differential scanning calorimetry, respectively. The MOF loading in Pebax®1657 increased the ideal H2/N2 selectivity from 6 to 8 thanks to an increased H2 permeability. In general, the MOF had little effect on the Pebax®165 membranes because an increase in gas solubility was neutralized by an equivalent decrease in effective diffusivity. Instead, the addition of MOF to PEEK-WC increases the ideal CO2/CH4 selectivity from 30 to ~48 thanks to an increased CO2 permeability (from 6 to 48 Barrer). The increase in CO2 permeability and CO2/CH4 selectivity is maintained under mixed gas conditions.