Journal: ChemCatChem 2021, 13, 1195–1200
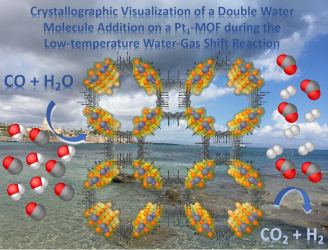
Abstract: The low-temperature water-gas shift reaction (WGSR, CO+H2O ⇔ H2+CO2) is considered a very promising reaction -candidate for fuel cells- despite an efficient and robust catalyst is still desirable. One of the more prominent catalysts for this reaction is based on single Pt atoms (Pt1) on different supports, which are supposed to manifold the reaction by the accepted mechanism for the general WGSR, i. e. by addition of one H2O molecule to CO, with generation of CO2 and H2. Here we show, experimentally, that not one but two H2O molecules are added to CO on the Pt1 catalyst, as assessed by a combination of reactivity experiments with soluble Pt catalysts, kinetic and spectroscopic measurements, and finally by in-operando single crystal X-ray diffraction on a Pt1-MOF, to visualize the formation of the hemiacetal intermediate on the solid catalytic site. These
results confirm our previous DFT predictions and provide a paradigmatic shift in the assumed mechanism of the WGSR, which may open the debate if two H2O molecules are recurrently added during the WGSR, not only for Pt1 catalysts but also for other metal catalysts.